- Index
- Brand
- Gross Weight
- Packing Size
- Power
- Voltage
- 110 V (51)
- 110-220v 50-60hz (8)
- 110v (371)
- 110v / 220v (4)
- 110v / 50hz / 60hz (11)
- 110v / 60hz (171)
- 110v / 60hz ± 10% (63)
- 110v / 60hz ± 10% (3)
- 110v / 60hz±10% (3)
- 110v 50hz (4)
- 110v Or 220v (3)
- 110v±10% (11)
- 110v, 50hz (2)
- 220v (2)
- Ac 110v (4)
- Ac 110v / 60hz (259)
- Ac100-120v, 60hz (2)
- Ac100v-240v 50-60hz (2)
- Ac110v / 50hz (3)
- Dc30v (3)
- Other (1949)
- Weight
US ECG90A Digital 12-Lead 12 channel ECG EKG Machine Electrocardiograph, Software
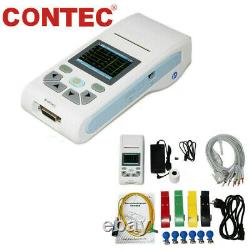

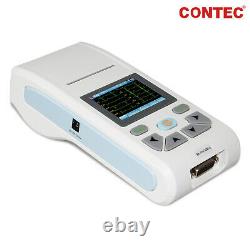










ECG90A Digital 12-Lead 12 channel ECG EKG Machine Electrocardiograph, Software. ECG90A is an Electrocardiograph which can collect 12-lead ECG signal simultaneously and print waveform by the thermal printing system. It features in, recording and displaying ECG waveform in AUTO/Manual mode, measuring and diagnosing ECG waveform parameters automatically, prompting for Lead off and Lack of paper, multi-language interface, case database management. 1Sync collection for 12-lead ECG, adopt digital signal processing technology and get high-quality ECG waveform via power frequency filter, baseline filter and EMG filter of ECG signal. 2Particular and high-accuracy digital filter eliminates baseline drift, no distortion to ECG waveform, greatly enhances anti-baseline drift capacity, convenient for waveform judgment. 3Display of 1/3/6/12-lead ECG, print mode, sensitivity, paper speed and filter state, etc. On one screen, convenient for contrastively diagnosing. 4Real-time and continuously record clear and accurate ECG waveform and annotation characterincluding lead mark, sensitivity, paper speed and filter state, etc. 5Adopt high-resolution thermal printing system, no need for any adjustments. Recording frequency: up to 150 Hz. 6With the functions of auto-analysis and auto-diagnosis for routine ECG parameters, provide measurement results and auto-diagnosis conclusion for HR, P-R interval, P Duration, QRS Duration, Q-T interval, Q-Tc, P Axis, QRS Axis, T Axis, R(V5), S(V1), R(V5)+S(V1), etc. Which reduces the doctors burden. 7Optional SD card stores up to 100 cases, which is convenient for doctor to review case and statistic. 8In review mode, case waveform stored can be checked, measurement parameter and diagnosis information can be automatically analyzed. 9Multi- language(Chinese, English) interface and report.
10Optional ECG-Sync software, connect with PC by USB2.0 interface to form a ECG workstation, import real-time data to the software for analyzing and report printing, and old cases stored in Electrocardiograph can be uploaded to PC. 11Power supply: AC/DC and built-in rechargeable lithium battery.
In optimal DC state, up to 4-hour standby time, continuous print 90-minute and 150-ECG, which meets the requirements of visiting a patient at home and body examination. Input mode: floating and defibrillation protection. Rated input amplitude Input frequency and waveform Relative output response. 1.0 0.67Hz40Hz, Sine wave ±10%a. 0.5 40Hz100Hz, Sine wave +10 %, -30 %a.
0.25 100Hz150Hz, Sine wave +10 %, -30 %a. 0.5 150 Hz 500 Hz, Sine wave +10 %, -100 %a.
1.5 1Hz, 200ms, Triangle wave +0 %, -10 %b. A relative to 10Hz b relative to 200 ms. Filter: power frequency(AC50/60 Hz), EMG25 Hz/35 Hz (-3 dB), baseline drift filter. Recording way: thermal printing system. Specification of recording paper: 50 mm(W)×20 m(L) high-speed thermal paper.Time base selection(paper speed): 6.25 mm/s, 12.5 mm/s, 25 mm/s, 50 mm/s, error: ±5%. Gain control(sensitivity): 2.5, 5, 10, 20 mm/mV, accuracy is ±2%; Standard sensitivity: 10 mm/mV±0.2 mm/mV. Recording mode: auto mode, rhythm mode and manual mode. Measurement parameters: HR, P-R interval, P Duration, QRS Duration, T Duration, Q-T interval, Q-Tc, P Axis, QRS Axis, T Axis, R(V5), S(V1), R(V5)+S(V1). Safety classification: Class I, type CF defibrillation-proof applied part.
ECG signal input sampling frequency: 32 kHz. Waveform data processing sampling frequency: 1 kHz. The minimum detection signal: 10 Hz, 20 µV(peak-peak value) deflected sinusoidal signal can be detected. Pacing detection channel: standard II. Accuracy of input signal: ±5%.
DC: 7.4V/2000mAh rechargeable lithium battery. Degree of protection against ingress of liquid: IPX0.
Dimension: 207mm(L)×96mm(W)×62mm(H). Food and Drug Administration and state and local regulatory agencies. The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG)with the code 197923, and certified by FDA of United States and CE, TUV of Europe. The Fingertip Pulse Oximeter that is FDA 510K Approved.
You pay us what you see on the invoice, i. Or replace item for you free of charge. Contec Medical Systems focusing on research, manufacture and distribution of medical instruments, was founded in 1992 as a high-tech company. At present there are more than 1200 employees in our company. Our product line covers a wide range of 13 categories. Most of the domestic hospitals are our customers.Contec hopes to cooperate with international companies to supply more innovative design and advanced technology products. We sincerely welcome you to become one of our global partners. We are looking forward to establishing a successful business relationship with you.
The item "US ECG90A Digital 12-Lead 12 channel ECG EKG Machine Electrocardiograph, Software" is in sale since Monday, July 29, 2019. This item is in the category "Business & Industrial\Healthcare, Lab & Dental\Medical & Lab Equipment, Devices\ECG & EKG Machines". The seller is "vmedshop0202" and is located in Elk Grove Village, Illinois. This item can be shipped to United States.- Number of Channels: 12
- ECG & EKG Machine Type: Electrocardiograph
- Model: ECG90A
- Country/Region of Manufacture: China
- Bundle Listing: Yes
- Operation: Digital
- MPN: 69450401
- Brand: CONTEC

